A unique tumour atlas will accelerate personalized cancer therapies by answering why cancers respond differently to treatments and streamlining the discovery and development of new approaches. Created by IMMUcan, a European public-private consortium co-led by SIB, the atlas integrates clinical data from over 2,700 patients along with high-quality molecular and imaging datasets for their tumours generated by cutting-edge technologies. Researchers and pharma companies can securely access and analyse the atlas – and digitally validate treatment hypotheses – through Switzerland’s national trusted research environment, BioMedIT.
IMMUcan stands for ‘Integrated iMMUnoprofiling of large adaptive CANcer patient cohorts’. Its 34 partners include academic institutions, clinical centres, patient organizations, and SME and pharmaceutical companies. The project, which started in 2019 and will end in 2026, receives support from the European Union within the Innovative Medicine Initiative 2 program and from the European Federation of Pharmaceutical Industries.
Identifying new options for patients with high-risk cancers
Cancer treatments have significantly improved over the past decade, particularly thanks to a revolutionary approach that harnesses the body’s immune system to attack tumour cells. However, not all cancers respond to these immunotherapies, and most acquire resistance over time.
IMMUcan stands for ‘Integrated iMMUnoprofiling of large adaptive CANcer patient cohorts’. Its 34 partners include academic institutions, clinical centres, patient organizations, and SME and pharmaceutical companies. The project, which started in 2019 and will end in 2026, receives support from the European Union within the Innovative Medicine Initiative 2 program and from the European Federation of Pharmaceutical Industries.
The EU-funded IMMUcan consortium aims to uncover the reason for immunotherapy resistance and identify effective treatments for patients with such high-risk cancers. To do so, its 34 partners are:
- generating and profiling comprehensive, high-quality data from over 2,700 patients across 80 European hospitals;
- integrating these data into a secure tumour atlas;
- analysing the data to understand how cancer and immune cells interact and the impact of current therapies.
The ultimate goal is to predict which patients will respond best to which treatments, and to develop precision oncology and personalized cancer therapies.
IMMUcan is co-led by SIB along with Merck, the European Organisation for Research and Treatment of Cancer (EORTC), Gustave Roussy and Bayer. Our Vital-IT Computational Biology group leads the development of a dedicated knowledge management system to store and share data and results, as well as work to identify biomarkers for predicting treatment outcomes. The group also co-coordinates the large-scale, 7-year collaboration and provides data integration and bioinformatics expertise to other work areas. The ambitious project contributes to SIB’s mission to accelerate innovation in medicine and health through in-depth knowledge of biological data, cutting-edge technologies, and interdisciplinary collaborations.
Generating comprehensive patient data
The project reached a major milestone late last year, with enrolment completed for patients with five types of cancer – breast, colorectal, lung, renal, and head and neck – who have received various treatments. Samples of their tumours and blood are being collected and stored in a state-of-the-art biobank, and extensively profiled using standardized protocols.
The profiling includes gene expression and mutation analyses, microscope imaging of tumour pathology, and immune cell profiling by two powerful techniques (immunofluorescence imaging and imaging mass cytometry, or IMC) that identify the position of individual cells in and around a tumour (the tumour microenvironment). The resulting molecular, cellular, and spatial data are then being integrated with clinical data like patient treatment histories and outcomes.
This massive effort includes more comprehensive patient cohorts than previous cancer profiling projects, and is the first to:
- focus on advanced-stage cancer;
- include patients who received recent standard-of-care treatments like immunotherapy;
- perform such comprehensive profiling, including of samples collected before and after treatment and the largest collection of IMC single-cell analyses of tumours to date;
- ensure high-quality data;
- support trustworthy AI with real-life, structured data.
Integrating and storing complex and sensitive data
SIB’s Vital-IT Computational Biology group led the design and development of the project’s knowledge management system for describing, harmonizing, integrating, and securely storing the vast amount of patient data generated and compiled by the project. This included standardizing drug names and other treatment information, developing automated tools for processing patient data, and establishing a secure database for managing, analysing and sharing the complex datasets and results without compromising patient privacy.
The resulting tumour atlas also includes a portal where IMMUcan partners can access, search, visualize, and analyse the data. The work drew on the group’s previous experience in large-scale European projects for diabetes, such as the Innovative Health Initiative’s Diabetes Platform (IMIDIA) and RHAPSODY study.
Accelerating new therapies through secure data analysis
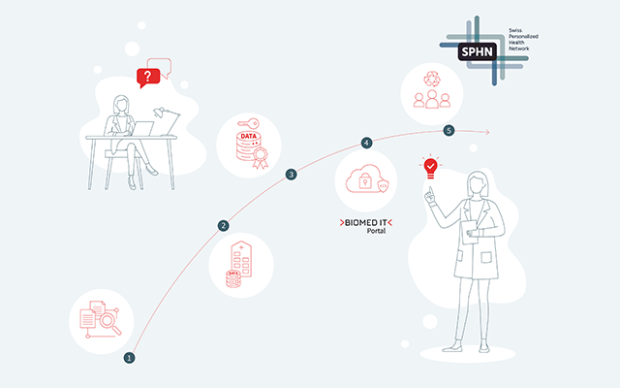
The tumour atlas is hosted on BioMedIT, the Swiss national trusted research environment coordinated by SIB for processing health-related data. Through controlled access, IMMUcan partners are studying the de-identified data to uncover how the tumour microenvironment influences current therapies, identify more targeted treatment options, and validate treatment hypotheses before initiating costly and time-consuming clinical trials – including through AI analyses.
As part of this, the Vital-IT Computational Biology group is performing advanced statistical analyses to identify which tumour characteristics could potentially serve as biomarkers to predict treatment outcomes. The group is also comparing data from the five tumour types to identify common and distinct traits.
The results of all analyses and data – as well as remaining biological samples – will eventually be made available for other research projects, under the same controlled access that respects patient consent and privacy.