A new collaboration framework allows private partners to transform sensitive Swiss health data into actionable insights while preserving patient privacy. The first pilot project examines risk factors for cardiovascular disease with Novartis.
Hospitals in the Swiss Personalized Health Network (SPHN) host a wealth of real-world data from over 700,000 patients who have given their general consent. With the new framework for public-private collaborations, pharmaceutical companies can answer simple research questions without accessing personal health data. These results can be translated into solutions for patients and, as such, further improve healthcare.
As the first project of this kind, SPHN partnered with Novartis along with the Cantonal Hospital Aarau and the University Hospital Basel. The framework for this collaboration was elaborated by SPHN with legal expertise from SIB.
About the Swiss Personalized Health Network (SPHN)
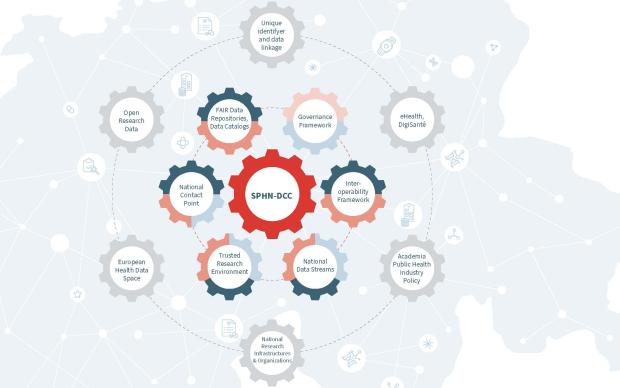
SPHN makes health data interoperable and shareable for research in Switzerland. It is coordinated by the Swiss Academy of Medical Sciences (SAMS) in collaboration with SIB. SIB notably provides the technical unit of SPHN to implement Swiss-wide FAIR data standards, and coordinates BioMedIT, the national IT platform enabling secure health-data exchange and processing for research.
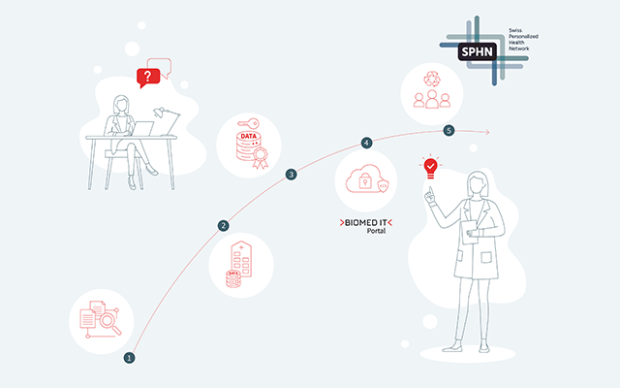
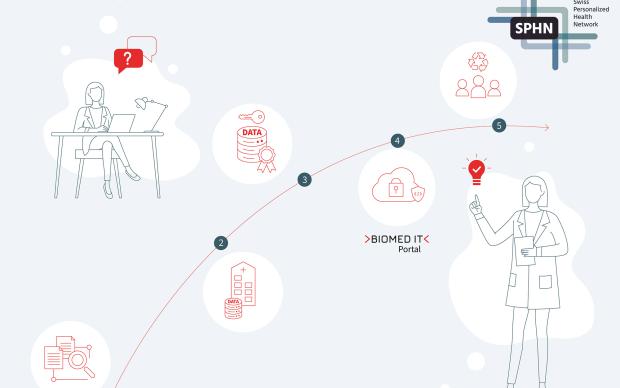
SPHN securely analyses sensitive data on behalf of the partner
The collaboration process is as simple as it is privacy-preserving:
About the Swiss Personalized Health Network (SPHN)
SPHN makes health data interoperable and shareable for research in Switzerland. It is coordinated by the Swiss Academy of Medical Sciences (SAMS) in collaboration with SIB. SIB notably provides the technical unit of SPHN to implement Swiss-wide FAIR data standards, and coordinates BioMedIT, the national IT platform enabling secure health-data exchange and processing for research.
- Novartis submitted a research question concerning a risk factor for heart disease;
- the hospitals transferred the necessary patient data in anonymized form to the Trusted Research Environment BioMedIT, where SPHN’s Data Coordination Center conducted the analysis;
- SPHN delivered aggregated results back to Novartis.
The project looks at lipoprotein, a blood protein that can raise the risk of cardiovascular disease. Measuring its levels in patients may help guide medication to prevent illness. As only summarized results are shared with the company, the project complies with the high ethical standards of SPHN for responsible data sharing and public-private partnerships.
A collaboration framework meeting industry, hospital and patient needs
With this new framework for collaboration with private partners, SPHN meets a need of both industry and hospitals, and caters to the strong wish of patients to enable research adhering to high ethical and data protection standards. As the public-private collaboration framework is now set up, more projects are expected to follow.