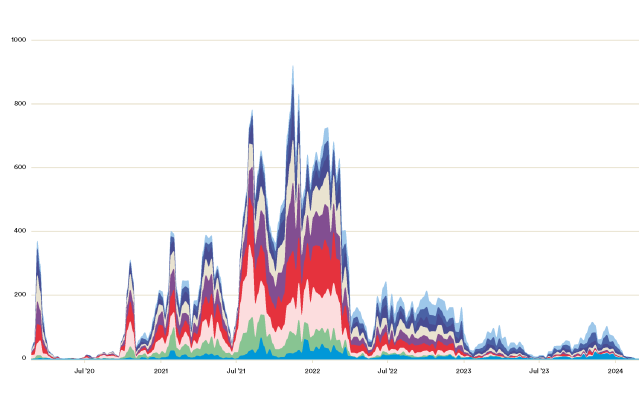
Public health authorities invest in surveillance programmes to monitor potentially threatening pathogens, research and mitigate the risks of outbreaks. In Switzerland, the Swiss Pathogen Surveillance Platform (SPSP), co-led by SIB, centralises microbial genetic sequences of public health interest collected in the country. In 2021, SPSP was mandated by the Federal Office of Public Health (FOPH) to collect, process and annotate the SARS-CoV-2 genomic data. The scope of its mission has since been expanded to include new pathogens, both for FOPH and the Federal Food Safety and Veterinary Office (FSVO).
Focus on SPSP
The Swiss Pathogen Surveillance Platform (SPSP) is a secure online platform that enables near real-time sharing under controlled access of pathogen molecular data and their associated clinical and epidemiological metadata.
Its two main objectives are:
- Surveillance: serve as a shared surveillance platform between human and veterinary medicine, including environmental and food isolates, thereby enabling detailed transmission and outbreak surveillance of pathogens in near real-time, with actionable results for public health.
- Research: serve as a FAIR repository of structured, standardized and well-annotated data that can be used to answer specific research questions; share data openly where possible.
A One Health approach at a national level
SPSP is a shared secure molecular surveillance platform between human and veterinary medicine which also includes environmental and foodborne pathogens. It is managed by SIB together with the University Hospitals of Basel, Lausanne and Geneva, as well as the Universities of Bern and Zurich. For SIB Associate Group Leader and Chair of the SPSP Scientific Board Aitana Neves, “SPSP serves as a crucial platform for the molecular monitoring of microorganisms occurring in humans, animals and the environment, fostering a One Health approach at a national level.”
Focus on SPSP
The Swiss Pathogen Surveillance Platform (SPSP) is a secure online platform that enables near real-time sharing under controlled access of pathogen molecular data and their associated clinical and epidemiological metadata.
Its two main objectives are:
- Surveillance: serve as a shared surveillance platform between human and veterinary medicine, including environmental and food isolates, thereby enabling detailed transmission and outbreak surveillance of pathogens in near real-time, with actionable results for public health.
- Research: serve as a FAIR repository of structured, standardized and well-annotated data that can be used to answer specific research questions; share data openly where possible.
Bringing Swiss public health authorities at the table
SPSP was mandated by the FOPH during the pandemic to collect, process and annotate SARS-Cov-2 genomic data. In 2023, two new endeavours for the Swiss authorities started to extend SPSP’s mandate on further pathogens of interest:
- Influenza and Respiratory Syncytial Virus (RSV), with a renewed funding from the FOPH, initially on human strains but already working with other labs, including Vetsuisse, on integrating animal strains;
- Listeria monocytogenes, Salmonella and more, with a funding from FOPH and the Federal Food Safety and Veterinary Office (FSVO).
SPSP is now an element of the FOPH’s infectious diseases dashboard, and is set to foster exchanges with other European pathogens platforms and to become a pole for pathogen genomics data, from animal, environmental and human sources.
“During the pandemic, SPSP has become a trusted partner of the FOPH, demonstrating the importance of genomic data curation and management to efficiently support national surveillance programs. It is essential that such infrastructures are consolidated to support current and future surveillance programs”, says Katrin Schneider, Head Bioinformatics and Data Science Unit at the FOPH.
Find out how SIB is co-steering European efforts to foster open sharing of SARS-CoV-2 genomic data
Reference(s)
Image legend: SARS-CoV-2 sequences deposited to the Swiss Pathogen Surveillance Platform: repartition by age of samples since 24 February 2020.