Focus on the group's mission
SIB Clinical Bioinformatics, led by Valérie Barbié, provides expertise and support for the organization, analysis and interpretation of patient and health-related data (e.g. omics data), converting them into clinically useful information for health professionals in order to foster optimal patients' care. Discover recent examples of our work as well as our expertise below.
The group notably contributes to:
- Addressing industry needs through innovation funding and leveraging expertise in AI, software development and quality control; Establishing state of the art diagnostic tools for Swiss hospitals and clinics;
- Developing national and international collaborative platforms to support clinical research, surveillance and diagnostic;
- Providing clinical bioinformatics training across Switzerland.
Addressing industry needs
The group partners with biotech or pharma industry on establishing new bioinformatics tools in various fields, e.g. imaging or genotyping. Recent examples include:
With the spatial biology company Lunaphore, an initial Innosuisse grant enabled a collaboration with the Geneva University Hospitals. The aim was to generate machine learning solutions to power the analysis of molecular imaging data to support the characterization of tumour microenvironments (read more). In 2023, a new collaboration, thanks to a second Innosuisse grant, started to develop AI-based assay development tools to enhance the automated approach of Lunaphore’s COMET™ platform, and to further accelerate spatial biology adoption in any research project.
Read more about Lunaphore & SIB collaboration
- For a Swiss pharmaceutical company, development of a software to monitor the genetic stability (absence of unwanted mutations) of bacterial strains using microfluidic DNA chips throughout the drug substance research and manufacturing process.
- Commercializing Melanie, SIB’s off-the-shelf software for the analysis of 2D electrophoresis gel and blot images. Its applications range from supporting the discovery of protein biomarkers and monitoring the adaptation of organisms to their environment to the development of diagnostic tests, quality control of food samples, and development of HCP ELISA tests (choice and validation of immunoassay reagents).
- Partner of the Human Lean Diagnostics (HLD) hub if Microcity (Neuchâtel’s innovation pole), which aims to support medical diagnostics and HealthTech by fostering collaboration and leveraging cantonal microtechnology expertise.
State-of-the-art diagnostic tools for hospitals
Through partnerships with hospitals, the group develops sample-to-report diagnostic tools that enable to streamline and automate bioinformatics analysis of patient data. Recent examples:
Developed the sample-to-report tool OncoBench®, with the Geneva University Hospitals (HUG). It is used by HUG Molecular Pathology since 2016 for the management and analysis of NGS patient data in routine cancer diagnosis (currently in version 5). This secure web-based platform streamlines and automatizes the complex molecular analyses leading to the diagnosis of cancer, while ensuring patient’s data privacy, and adhering to hospital quality assurance standards. This type of platform can be used for molecular pathology diagnostics in pathology laboratories.
Example of a data flow in personalized medicine supported by SIB’s expertise:
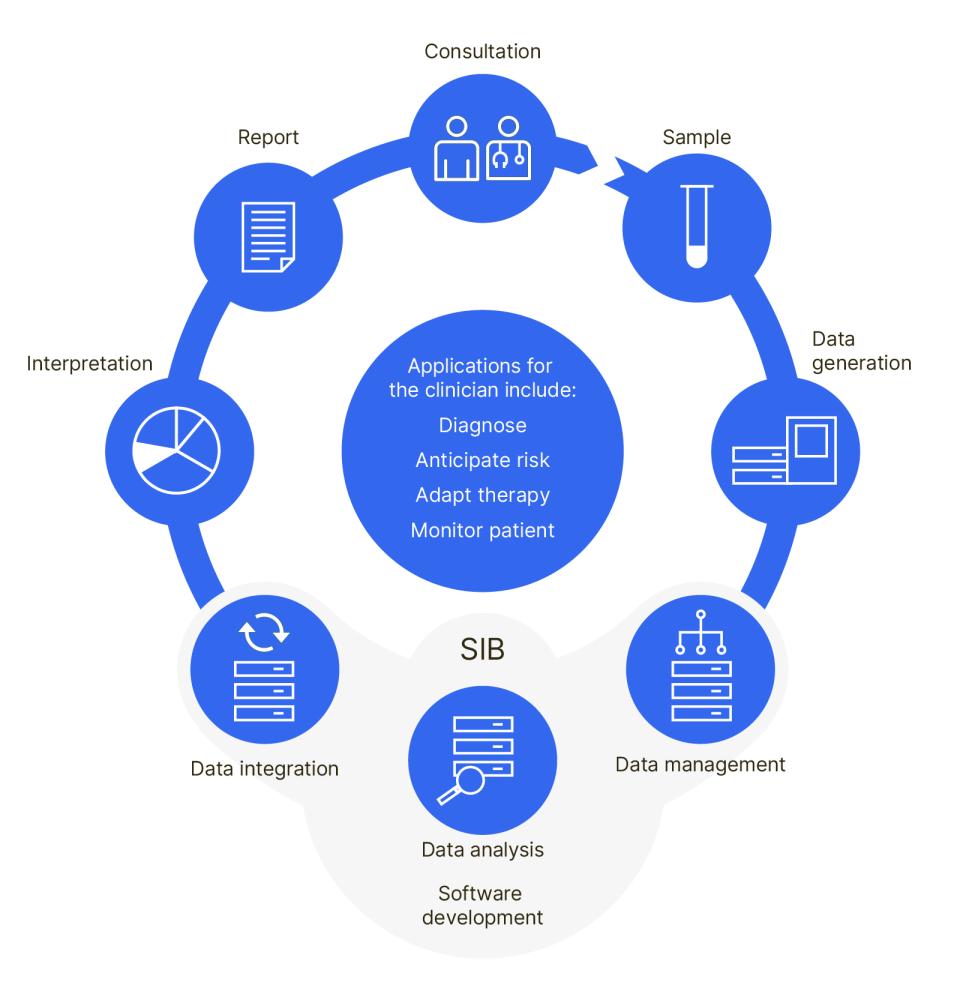
Supporting nationwide clinical research and surveillance
The group develops national collaborative solutions to support omics research, clinical diagnostic and pathogen surveillance. For the latter, it does so through SIB’s Centre of Pathogen Bioinformatics. Recent examples include:
- Co-leading with several hospitals and veterinary institutions the Swiss Pathogen Surveillance Platform (SPSP), the national One Health genomic platform to support pathogen surveillance and research. The challenges it addresses include: ensuring data compliance with Swiss Personalized Health Network (SPHN) standards, implementing bioinformatics analyses, and managing controlled data access for users such as clinical microbiology laboratories, researchers, and public health authorities. SPSP is an SIB Resource and is funded by the State Secretariat for Research and Education and the Federal Office of Public Health.
- Co-leading the initial setup of the SwissGenVar platform, a nation-wide effort connecting for the first time all major academic institutions for Medical Genetics in Switzerland. Its aim is to integrates diagnostic-grade genetic variants interpretation and high-quality clinical data (initially funded by SPHN).
Providing clinical bioinformatics training across Switzerland
Training in clinical bioinformatics for clinical professionals contributes to enhanced communication across experts from various disciplines and better use of these novel technologies in daily routine:
- A Certificate of Advanced Studies (CAS) in Personalized Molecular Oncology is coordinated since 2018 by the group in collaboration with the University Hospitals of Basel and Lausanne, and the University of Basel. First of its kind in Switzerland, this multi-disciplinary and multi-site programme aims at training the next generation of professionals from various backgrounds in this rapidly evolving field.
Our expertise
For the projects we have worked on, we leverage a wide range of know-how, including:
- Project and product management;
- Business analysis;
- Scientific computation and analysis;
- Data management;
- Bioinformatics analysis;
- Machine learning and signal/image analysis;
- Software engineering.
Molecular Big Data, a new weapon for medicine - Experts will meet in Switzerland in September: “Pathogen sequencing to track epidemics” RTS CQFD
Precision medicine and the role of bioinformatics: four short films to understand: Canal9 (short film)
Molecular medicine and the CAS in Personalized Molecular Oncology: Bulletin des médecins suisses
- F. Psomopoulos et al.
Toward a unified approach: Considerations for bioinformatic and sequencing activities & data in wastewater surveillance of biologic public health threats.
Open Res Europe 2025, https://open-research-europe.ec.europa.eu/articles/5-267/v1 - M. Ventouratou et al.
The Network of National COVID-19 Data Portals: public health equity through collaboration.
Open Res Europe 2025, https://doi.org/10.12688/openreseurope.20059.1
- Y. Christinat et al.
Reporting of somatic variants in clinical cancer care: recommendations of the Swiss Society of Molecular Pathology.
Virchows Archiv, https://doi.org/10.1007/s00428-024-03951-0 - E. Mutschler et al.
Towards unified reporting of genome sequencing results in clinical microbiology.
PeerJ 12:e17673. https://doi.org/10.7717/peerj.17673 - D. Kraemer et al.
SwissGenVar: A Platform for Clinical-Grade Interpretation of Genetic Variants to Foster Personalized Healthcare in Switzerland.
J. Pers. Med. 2024, 14, 648. https://doi.org/10.3390/jpm14060648 - F. Wegner et al.
How much should we sequence? An analysis of the Swiss SARS-CoV-2 surveillance effort.
Microbiol Spectr. 2024, doi: 10.1128/spectrum.03628-23.
- Aitana Neves et al.
A survey into the contribution of regional/national pathogen data platforms and on the resources needed to develop and maintain them
F1000Research 2023, doi: 10.12688/f1000research.142165.1 - Aitana Neves et al.
FAIR+E pathogen data for surveillance and research: lessons from COVID-19
Frontiers in Public Health, doi: 10.3389/fpubh.2023.1289945 - Aitana Neves et al.
The Swiss Pathogen Surveillance Platform – towards a nation-wide One Health data exchange platform for bacterial, viral and fungal genomics and associated metadata.
Microbial Genomics, doi: 10.1099/mgen.0.001001
- JJC de Vries et al.
Benchmark of Thirteen Bioinformatic Pipelines for Metagenomic Virus Diagnostics Using Datasets from Clinical Samples.
Journal of Clinical Virology : The Official Publication of the Pan American Society for Clinical Virology, doi: 10.1016/j.jcv.2021.104908 - JJC de Vries et al.
Recommendations for the Introduction of Metagenomic Next-Generation Sequencing in Clinical Virology, Part II: Bioinformatic Analysis and Reporting.
Journal of Clinical Virology : The Official Publication of the Pan American Society for Clinical Virology, doi: 10.1016/j.jcv.2021.104812
- G. Greub et al.
Clinical Bioinformatics for Microbial Genomics and Metagenomics: An ESCMID Postgraduate Technical Workshop.
Microbes and Infection, doi: 10.1016/j.micinf.2020.07.008 - D. Dylus et al.
NGS-Based S. aureus Typing and Outbreak Analysis in Clinical Microbiology Laboratories: Lessons Learned From a Swiss-Wide Proficiency Test.
Frontiers 2020, doi: 10.3389/fmicb.2020.591093 - A. David et al.
Annotation and curation of human genomic variations: an ELIXIR Implementation Study.
F1000Research 2020, doi: 10.12688/f1000research.24427.1
- T. Junier et al.
Viral Metagenomics in the Clinical Realm: Lessons Learned from a Swiss-Wide Ring Trial.
Genes 2019, doi: 10.3390/genes10090655
- A. Egli et al.
Improving the quality and workflow of bacterial genome sequencing and analysis: paving the way for a Switzerland-wide molecular epidemiological surveillance platform.
Swiss Med Wkly 2018, doi: 10.4414/smw.2018.14693 - D. Wüthrich et al.
Modern Microbiological Surveillance for Antibiotic Drug Resistance.
Swiss Antibiotic Resistance Report 2018, FOPH publication number: 2018-OEG-87, 93-95 - D. Stekhoven et al.
Swiss Variant Interpretation Platform for Oncology (SVIP-O).
Swiss Med Informatics. 2018, doi: https://doi.org/10.4414/smi.34.00411 - V. Barbié et al.
La santé de demain déjà à la portée des médecins.
Bulletin des Médecins Suisses 2018, doi: https://doi.org/10.4414/bms.2018.06782
- SIB Swiss Institute of Bioinformatics members
The SIB Swiss Institute of Bioinformatics\' resources: focus on curated databases.
Nucleic Acids Res. 2016, doi: 10.1093/nar/gkv1310 - VB Gerritsen et al.
Bioinformatics and personalized medicine: Switzerland is pioneer.
Rev Med Suisse 2016; 12 : 414-6 - JS. Beckmann et al.
Reconciling evidence-based medicine and precision medicine in the era of big data: challenges and opportunities.
Genome Medicine (2016), doi: 10.1186/s13073-016-0388-7
Members
View our group members here